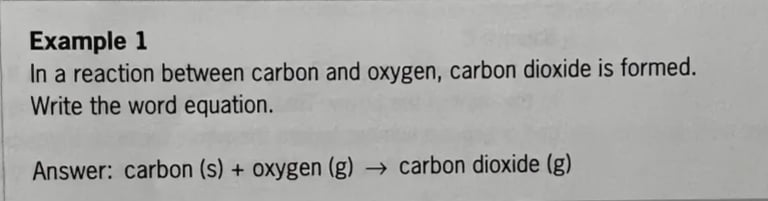Word equation
In chapter 1 you summarised a phase transition in a diagram. The evaporation of water, for example, can be given as: water () → water (g). If you do that for a chemical reaction, this is called a word equation. A word equation shows the reactants to the left of the arrow and the reaction products to the right of the arrow. After each substance, the phase of that substance (solid, liquid or gas) is indicated in abbreviated form.
In addition to solid, liquid and gaseous substances in a reaction, substances can also be dissolved (as a solution). If nothing else is mentioned, 'dissolved' in chemistry always means 'dissolved in water'. An abbreviated state designation has also been devised for this: (aq). This is derived from the Latin word for water: aqua. Below are the four indications of the state listed that you need to know. They are followed by the abbreviation that you should use in word equations.
solid: (s)
liquid: ()
gaseous: (g)
dissolved: (aq)