Characteristics of a chemical reaction
Reaction temperature
Not all reactions take place at room temperature. That is a good thing; if that was the case, a book would ignite spontaneously, and you could no longer use it. For every reaction a minimum temperature is needed at which the reaction will proceed. This temperature is called the reaction temperature. The reaction temperature for igniting paper is higher than room temperature. Paper only ignites from 200°C. Energy effect
Energy effect
When heating the magnesium ribbon, you see a bright white light. This reaction also produces energy in the form of heat. Reactions that produce energy, are called exothermic reactions. Combusting wood and natural gas are also exothermic reactions; in both cases energy in the form of heat is produced. These combustion reactions do have a high reaction temperature. They do not take place spontaneously, but you have to light the fuel. Only when the reaction has started, energy is produced. The amount of energy produced is greater than the amount of energy needed to start the reaction.
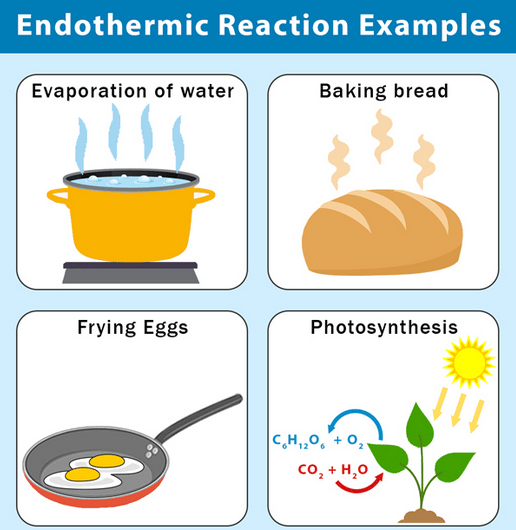
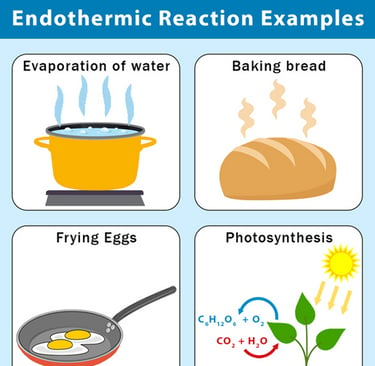
There are also reactions that require a continuous supply of energy, these are called endothermic reactions. For example, if you get a white filing in your molar, the dentist will use light to initiate an endothermic reaction in which the filling will harden, see figure 3.2. When the dentist stops the exposure, the reaction will also stop immediately. The energy required or released during a reaction is called the energy effect.