3.1 Characteristics of chemical reactions
4. Explain in which of the following processes there is a chemical reaction:
a) the melting of candle grease
b) the explosion of dynamite
c) dissolving sugar in your tea
d)the discolouration of ink by sunlight
e)the removal of nail polsih with a nail polish remover
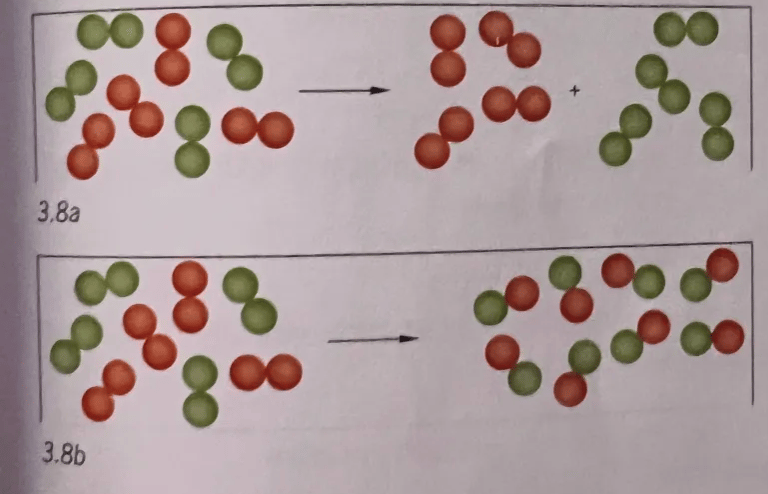
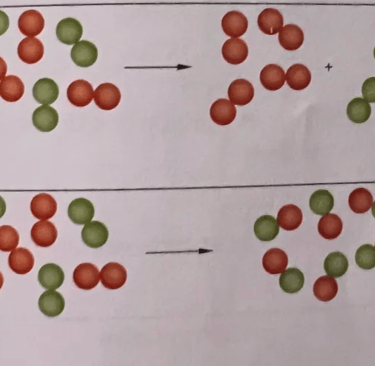
5. look at figure 3.8a and b. Each dot represents an atom.
a) Explain which drawing represents a chemical reaction
b)Explain that both processes have conservation of mass
6 a)What is the difference between a normal exothermic reaction and an explosion?
b)Explain which of the five factors that influence the rate of a reaction determines whether a dust explosion will occur
7. If you drop a grain of the metal calcium into enough water, the grain will disappear: a colourless solution and a gas are formed. Explain why this must be a chemical reaction
8. Food spoilage is a chemical reaction
a) Explain why food can be kept longer in the refrigerator
Combustion is a reaction with oxygen. IF you want to light a campfire. its best to start with thin twigs.
b) Explain why thin twigs will burn more easily than a large piece of wood
9. An energy effect also occurs with phase transitions
a) Explain whether the melting of candle grease is an exothermic or endothermic process
b) Explain whether condensing water is an exothermic or an endothermic process